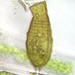
Photos / Sounds
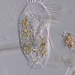
What
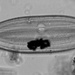
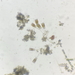
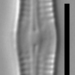
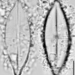
Aulacoseira ambiguaObserver
closterium_mysteriumDescription
Video: https://youtu.be/dikipnse9qQ
Sampling location: A water sample was taken from the bank of the Vuoksi River.
Date and time of collection: 22 Jul 2023 at 12 PM
Date and time of observation: 23 Jul 2023 at 1 PM
The sample was stored at room temperature in a plastic container.
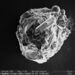
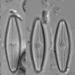
Photos / Sounds
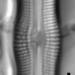
What
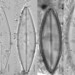
Genus NaviculaObserver
dgborinDescription
Length: 51 µm
Width: 11 µm
12 striae per 10 µm
Observer
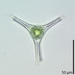
davidfbirdDescription
The purple sulfur bacteria plate at the chemocline in meromictic Mahoney Lake. There is a whole complex community in there, but the major component that makes the plate visible is apparently Lamprocystis purpurea. See Klepac-Ceraj, V. et al. (2012) Microbial diversity under extreme euxinia: Mahoney Lake, Canada. Geobiol. 10(3):223-35.
doi: 10.1111/j.1472-4669.2012.00317.x
Observer
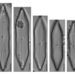
laneallenDescription
Scale Bar = 10 µm
Sample collected from a sediment core -4cm deep.
Burge, D., Edlund, M. (2017). Fragilariforma polygonata. In Diatoms of North America. Retrieved December 18, 2023, from https://diatoms.org/species/fragilariforma_polygonata
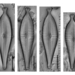
What
Actinella punctataObserver
laneallenDescription
Scale Bar = 10 µm
Sample collected from a sediment core -4cm deep.
Beals, J. (2011). Actinella punctata. In Diatoms of North America. Retrieved December 18, 2023, from https://diatoms.org/species/actinella_punctata
What
Genus FragilariformaObserver
laneallenDescription
Scale Bar = 10 µm
Sample collected from a sediment core -4cm deep.
NOT F. constricta.
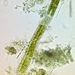
Photos / Sounds
What
Genus GyrosigmaObserver
davidfbirdDescription
160 µm long, on decayed bit of vegetation.
Photos / Sounds
What
Entomoneis ornataObserver
closterium_mysteriumDescription
A water sample was taken from the bank of the Vuoksi River. The sample was stored at room temperature and observed one day after collection.
Video: https://youtu.be/eGHWgdZYq3Q
Observation of Surirella librile https://www.inaturalist.org/observations/192091414
Photos / Sounds
What
Difflugia brevicollaObserver
bdstaylorDescription
An amoeba that builds its shell from found materials (xenosomes). This one incorporates diatoms, the spherical cysts of golden algae and tiny grains of sand.
Collected in the Mer Bleue Bog conservation area, air-dried on conductive tape and images in a scanning electron microscope at the Canadian Museum of Nature.
Photos / Sounds
What
Surirella librileObserver
closterium_mysteriumDescription
Fig. 1-3: Valve view
Fig. 4-6: Girdle view
GIF and Fig. 7-9: A few images as the diatom rotates
A water sample was taken from the bank of the Vuoksi River. The sample was stored at room temperature and observed one day after collection.
Video: https://youtu.be/n7HxWvJmyp4
Observation of Entomoneis ornata https://www.inaturalist.org/observations/192091414
Photos / Sounds
What
Genus GrammatophoraObserver
lamawebberDescription
Genus: Grammatophora Ehrenberg, C.G. (1840).
The type species (lectotype) of the genus Grammatophora is Grammatophora angulosa Ehrenberg (Guiry and Guiry 2023)
Taxonomic notes on the genera:
Phylum Bacillariophyta
Subphylum Bacillariophytina
Class Bacillariophyceae
Subclass Fragilariophycidae
Order Rhabdonematales
Family Grammatophoraceae
Found on Z. marina at Montague Harbour Marine Provincial park (MHMPP)
Taxonomic characteristics of the genus:
- An araphid genus, they often form zig-zag colonies attached to marine substrates
- In girdle view the cells appear rectangular to square
- In valve view the cells are elongate with rounded apices and no distinct central area
- The girdle bands, closet to the valves (valvocopula) display distinctive undulating septa
- The septa, either highly undulate or straight run from the apex of the girdle band nearest the valve (valvocopula) and terminate close to the cell centre. Towards the centre, the septa sometimes hooks back towards the valve. As the cell decreases in size the septa change shape and become less undulate, except for the hooked end which is morphologically stable (Sato et al. 2010)
- Apical pore fields, where there are generally small spines
- Pseudosepta located at the apices
- Areolae are fine, poroid, uniserate and transverse, linearally arranged or five poroids arranged in a cross (quincunx pattern)
- Chloroplasts are four per cell, filling the space in the septa
- Two rimoportulae located close to the apices (Round et al. 1990, Sato et al. 2010, Spaulding et al. 2021)
Methods:
Collected by brushing or scrapping leaf sections of Z. marina from MHMPP, August 3, 2020, October 16, 2020, March 7, 2021 and July 2021. Cells were cleaned with concentrated hydrogen peroxide or nitric acid at 100 C for 3-5 hours to remove organics, then rinsed multiple times in ddH20 to a neutral pH. Mounted on SEM stubs or in Naphrax on slides for light microscopy. Imaging with a Nikon TE300 and Tuscen DigiRetna16 MP camera or Nikon E800.
Live specimens were imaged with either a Nikon TE300 or Nikon E800 with either bright-field or DIC. In-situ, environmentally prepared samples were made using minimal contact of 8-10 mm leaf section, soaked in ddH2O to remove salts and dried through an EtOH series (50%-100%) and finished off with 100% Hexamethyldisilane HMDS (Hazrin-Chong and Manefield 2012). Mounted on carbon stickies onto aluminum SEM stubs and imaged with either the Hitachi s4800 or TM4000 at AMF, at University of Victoria, B.C. My thanks to Siobhan Schenk and Laura Parfrey in the Parfrey Lab at UBC for molecular data from the eelgrass and a collaboration with IMERSS. Also thanks go to Elaine Humphrey of the AMF, UVIC, imaging by Ron Read, Melanie Quenneville and Arjan van Asselt. Imaging, taxonomy and identifications by M. Webber.
References:
Al-Yamani, F., and M. A. Saburova, M. (2011). Illustrated Guide on the Benthic Diatoms of Kuwait’s Marine Environments,” Kuwait Institute for Scientific Research, Lucky Press. p. 148. Plate 156. a-b.
Siqueiros Beltrones D. A., U. Argumedo Hernández and C. Landa Cancigno. 2015. Uncommon species diversity values in epiphytic diatom assemblages of the kelp Eisenia arborea. Hidrobiológica 26 (1): 61-76.
Guiry, M.D. in Guiry, M.D. & Guiry, G.M. 2020. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 4 November 2023.
Hazrin-Chong NH, Manefield M. (2012 ). An alternative SEM drying method using hexamethyldisilazane (HMDS) for microbial cell attachment studies on sub-bituminous coal. J Microbiol Methods. 90(2):96-9. doi: 10.1016/j.mimet.2012.04.014.
Mather et al. (2010). A Checklist of Diatom Species Reported (and Presumed Native) from Canadian Coastal Waters. Fisheries and Oceans Canada (https://publications.gc.ca/collections/collection_2010/mpo-dfo/Fs97-6-2881-eng.pdf).
Plinski, M. & Witkowski, A. (2020). Diatoms from the Gulf of Gdansk and surrounding waters (the southern Baltic Sea). pp. [1]-442, incl. 31 SEM pls, 16 photo pls. Gdansk: Gdansk University Press.
Rao, V.N.R. and Levin, J. 1976. Benthic marine diatom flora of False Bay, San Juan Island, Washington. Syesis, 9:173–213.
Round, F.E.,Crawford, R.M. & Mann, D.G. (1990), The Diatoms, Biology & Morphology of the Genera, pp. 436-437. Cambridge University Press, Cambridge, UK.
Sato, S., Nagumo, T. & Tanaka, J. (2003). Morphology and Taxonomy of Five marine attached diatoms in the genus Grammatophora Ehrenberg (Bacillariophyceae) in Japan. Japanese J. of Phycology 51 (supplement): 183-187.
Sato, S., Nagumo, T. and Tanaka, J. (2010): Morphological Study of Three Marine Araphid Diatom Species of Grammatophora Ehrenberg, with special reference to the septum structure
, Diatom Research, 25:1, 147-162. http://dx.doi.org/10.1080/0269249X.2010.9705835
Saunders, K., Lane, C., Cook, S., McMinn, A., & Hallegraeff, G. Benthic Diatoms: in Jameson, I. & Hallegraeff, G.M. (2010). Planktonic diatoms. In: Algae of Australia. Phytoplankton of temperate waters. (Hallegraeff, G.M., Bolch, C.J.S., Hill, D.R.A., Jameson, I, LeRoi, J.-M., McMinn, A., Murray, S., de Salas, M.F. & Saunders, K. Eds), pp. 122-123. fig 3.3A. Canberra & Melbourne: ABRS; CSIRO Publishing.
Shim, J. H. (1976). Distribution and Taxonomy of Planktonic Marine Diatoms in the Strait of Georgia, B.C. Phd. Thesis, UBC. p. 186. p. 246 Plate XXIV. FIG. 11.
Sims, P.A. (ed.) (1996). An atlas of British diatoms arranged by B. Hartley based on illustrations by H.G. Barber and J.R. Carter. pp. [2], 1-601, incl. 290 pls. Bristol: Biopress Ltd.
Tynni, R. (1986). Observations of diatoms on the coast of the state of Washington. Geological Survey of Finland, Report of Investigation 75.
Witkowski, A., Lange-Bertalot, H. & Metzeltin, D. (2000). Diatom flora of marine coasts I. Iconographia Diatomologica 7: 1-925, incl. 219 pls with 4504 figs.
Photos / Sounds
What
Genus TryblionellaObserver
mnold1Description
Mag. 100x (1,3,5), 400x (2,4,6)
Very large pennate diatom (common in this water sample); 3 specimens were photographed with lengths 520µ, 540µ, and 575µ. Images 1 and 2 suggest a clash of Titans; the 230µ long Pinnularia (perhaps P. pulchella) is less than half the length of its rival. Images 3-6 record the 2nd & 3rd specimens (dead; frustules more exposed) of this huge pennate diatom. roman_romanov speculates these giants belong to the genus Nitzschia or Tribonella (https://www.inaturalist.org/observations/124342691).
- After gently pushing aside the cover of lily pads, a pond side water sample was taken on 07/16/2022 using a 10µ dip net to enrich for microbes. Air temp. 79°F.
Photos / Sounds
What
Order CymbellalesObserver
closterium_mysteriumDescription
A water sample was taken from the bank of the Vuoksi River. The sample was stored at room temperature and observed one day after collection.
Photos / Sounds
What
Early Brown Spinner (Leptophlebia cupida)Observer
laneallenDescription
L. gravastella?
Photos / Sounds
Observer
closterium_mysteriumDescription
Observation of two specimens of Amphipleura from the same water sample, made within two days.
GIF and Fig.2 & 3 - first specimen
Fig.1 - second specimen
A water sample was taken from the shore of Srednerogatsky Pond. The air temperature was 14°C (57.2 °F). The sample was stored at room temperature and observed 4 days after collection.
Video: https://youtu.be/KNJsVGG4diY
What
Genus MelosiraObserver
manuel_helsinkiDescription
Found in a water sample from a beach in the Baltic Sea.
Photos / Sounds
What
Genus NeidiumObserver
damontigheDescription
Images taken with Bio-Rad ZOE fluorescent imager
What
Genus NeidiumObserver
laneallenDescription
Scale Bar = 10 µm.
Ask me about any of these taxa for more info.
Photos / Sounds
What
Genus LacrymariaObserver
aquoriaDescription
Lacrymaria (olor?) but has two heads functioning separately but together. @bdstaylor whhhat is going on here? (Thanks to @izi_izi for the sample containing this.)
Photos / Sounds
What
Asterionella formosaObserver
lamawebberDescription
Asterionella formosa Hassall 1850.
Phylum: Bacillariophycophyta, Subphylum: Bacillariophytina, Class: Bacillariophceae, Order: Rhabdonematales, Family: Tabellariaceae.
Holotype species: Asterionella formosa Hassall 1850 = A. gracillima (Hantzsch) Heiberg 1863 (Plinski and Witkowski 2020, Guiry and Guiry 2023)
In girdle view, typical radiate colony of 4 to 8 cells (Smith1850) and elongate, narrow, linear. They have unequally inflated capitate ends and the valves are distinctively heteropolar. The proximal end of the valve (foot pole) is attached to other cells in the colony. Cells tapering from the middle to the apices. The foot pole is slightly larger and distinctively capitate compared to the distal valve end. A transverse rimoportula is located at both apices. Areolae are uniserate and very fine; 24-28 (Patrick and Reimer 1966, Spaulding 2012 and Plinski and Witkowski 2020), this Howe Sound specimen is 30 in 10 µm. Striae are offset from one another at the very narrow central sternum. Apical pore field present at both poles. Irregular marginal spines may, or may not, be present (Spaulding 2012). Chloroplasts are numerous small plates. Size range: 40-130 µm long x 1-3 µm width (Patrick and Reimer 1966); 30-160 µm long x 1.3-6 µm wide (Plinski and Witkowski 2020); 45-68 µm long x 1.1-4.5 µm wide (freshwater) (Spaulding 2012). Howe Sound specimen: 76.4 x 2.4 µm. Environment; freshwater (Smith1850, Patrick and Reimer 1966, Sims 1996, Guiry and Guiry 2023); marine or estuarine (Mayama et al. 2018, Plinski and Witkowski 2020).
SEM images of Asterionella formosa found with the sponge Hymedesmia sp., Anvil Island, Howe Sound. B.C. Depth 60 ft. August 19, 2011. Specimen NM 267. Imaged by Ron Read on a Hitachi s4800 SEM. SEM specimen prepared by Elaine Humphrey, Advanced Microscope Facility (AMF), University of Victoria. From specimens collected by Bruce Ott (Royal British Columbia Museum) and Neil McDaniel (Neil McDaniel Marine Consulting). Taxonomy by MW.
References:
Guiry, M.D. & Guiry, G.M. 2007, AlgaeBase version 4.2. World-wide electronic publication, National University of Ireland, http://algaebase.org, searched April 10, 2022.
Mayama, S., Taguchi, Y. and Nomura, H. 2018. Diversity of diatoms and their community dynamics in Tama River estuary. The Yokyu Foundation for Better Environment, Grant Awarded Academic Researches Vol.47,No. 335:79 pp.
Pappas, J. L. and Stoermer, E. F. (2001). Asterionella Taxonomic history and quantitative methods as an aid to valve shape differentiation. Diatom. 17:47-58.
Patrick, R.M. and Reimer, C.W. (1966) The Diatoms of the United States exclusive of Alaska and Hawaii, V. 1 Monographs of the Academy of Natural Sciences of Philadelphia 13. p. 180 Plate 9, Fig. 6.
Plinski, M. and Witkowski. A. (2020). Diatoms from the Gulf of Gdansk and surrounding waters (the southern Baltic Sea. Gdansk University Press. Gdansk.
Round, F.E., Crawford, R.M. and Mann, D.G. (1990). The Diatoms, Biology & Morphology of the Genera. Cambridge University Press, Cambridge, UK. pp. 132-133.
Shim, J. H. (1976). Distribution and Taxonomy of Planktonic Marine Diatoms in the Strait of Georgia, B.C. Phd. Thesis, UBC. P. 165, Plate 21, fig. 1.
Sims, P.A. (ed.) (1996). An atlas of British diatoms arranged by B. Hartley based on illustrations by H.G. Barber and J.R. Carter. pp. 72-73, Fig. 1. Bristol: Biopress Ltd.
Smith, W. (1856). A synopsis of the British Diatomaceae; with remarks on their structure, functions and distribution; and instructions for collecting and preserving specimens. The plates by Tuffen West. In two volumes. Vol. II. A-E. London: John van Voorst, Paternoster Row.
Spaulding, S. (2012). Asterionella formosa. In Diatoms of North America. Retrieved May 16, 2023, from https://diatoms.org/species/asterionella_formosa.
Stoermer, E. F. (2002). Great Lakes Diatom Home Page, Center for Great Lakes and Aquatic Sciences, University of Michigan http://websites.umich.edu,/~phytolab/GreatLakesDiatomHomePage/Asterionella/Asterionellableakeleyi/Asterionellableakeleyicard.html
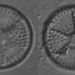
What
Genus CoscinodiscusObserver
laneallenDescription
Scale Bar = 10 µm.
Found on a parasitic macrophyte.
What
Genus ActinoptychusObserver
laneallenDescription
Scale Bar = 10 µm.
Found on a parasitic macrophyte.
What
Genus ActinoptychusObserver
laneallenDescription
Scale Bar = 10 µm.
Found on a parasitic macrophyte.
Observer
laneallenDescription
Scale Bar = 10 microns.
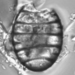
This specimen was collected from the bottom layer of a volcanic ash deposited approximately 11.8 million years ago. This specimen had secondary costae and a costae density of 3 per 10 microns. Valves are elliptical nearly circular. This specimen was identified using Schmidt's Plates and the Hustedt Collection which was collected from a deposit of approximately the same time frame in Oregon. The specimens did not correspond well to the dimensions published in Williams 1996; however, his dimensions did not match the figures published in the same paper. This form is distinguished by its diminutive size and nearly circular valves.
What
Terpsinoe musicaObserver
diatomwuDescription
Composite epiphytic and epilithic sample from the edge of the Trinity River.
Length: 116 um, Breadth: 35 um.
Characteristics: 3 inflations in larger specimens, a single rimoportula, and transapical bars that look like music notes in girdle view.
Photos / Sounds
What
Hippodonta capitataObserver
closterium_mysteriumDescription
A water sample was collected from the shore of Srednerogatsky Pond. The air temperature was 14°C (57.2 °F). The sample was kept at room temperature until it was assayed 7 days later.
Video: https://youtu.be/9ONVEXDLuEE
Photos / Sounds
Observer
mnold1Description
Mag. 400x
Proportions: 100µ x 22µ; 8-9 striae per 10µ at the center. The size and unique shape of this Pinnularia put it within the range reported for Pinnularia undula var, major as seen here https://diatoms.org/species/pinnularia-undula-var-major. (It is larger than visually-related P. undula). This species is currently not in the iNat database. The second image captures a transition from girdle view to valve view.
- A water sample was taken on 5/8/2023, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 73°F.
What
Life (Life)Observer
juanloredoDescription
Water sample collected from the river San Pedro, Rosales, Chihuahua, at the indicated location.
The samples was collected on January 13th and images were acquired next day.
Photos / Sounds
What
Odontella auritaObserver
woodilljDescription
- from marine sample (sand and water), collected at shoreline
- objective(s) (x 10): images 1-9, 1000x; image 10, 600x
- approximate dimensions: see image 11
- ID: ELPB-LGT40
Photos / Sounds
What
Genus FrustuliaObserver
laneallenDescription
Scale Bars = 10µm.
First image is of the iridescence produced by this taxon under total extinction DIC.
Photos / Sounds
What
Genus CymbopleuraObserver
mnold1Description
Mag. 400x
Freshwater, asymmetric diatom. The 3 photos in image 1 depict a small rotation around its longitudinal axis. Based on its uniquely large size (180µ L x 50µ W), apiculate and capitate apices, striae count per 10µ (7 at the valve center and 9 near the apices), this specimen (to my eyes) looks like Cymbopleura inequalis. For more information and reference photos see https://diatoms.org/species/cymbopleura_inaequalis and https://www.algaebase.org/search/species/detail/?species_id=q6409a9e46e8376c6.
- A water sample was taken on 11/08/2022, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 55°F.
Photos / Sounds
Observer
cygnetureDescription
Images 1-7
- Algae sampled just below pontoon water line.
- Filament structure.
- Filament structure.
- Cell structure.
- Filaments structure.
- Cell structure.
- Matted strands forming tufts.
Photos / Sounds
What
Sea Stars (Class Asteroidea)Observer
laneallenDescription
Scale Bar = 500µm.
Photos are courtesy of Jeffery Stone who let me pilot his SEM briefly yesterday. I am not sure where the population was originally from but this is a starfish that was in the Indiana State University marine aquarium.
What
Genus MastogloiaObserver
aliendiatomDescription
Mastogloia belaensis. Biscayne Bay Coastal Wetlands. Collected on flocculent plant material.
Photos / Sounds
What
Melosira variansObserver
pierrenoelDescription
Légende
A. Vue générale
B->H. Filaments stériles & cytologie
I->V. Auxospores
W-X. Lavoir / Wash-house.
Même place & habitat que https://www.inaturalist.org/observations/105116866
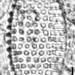
What
Diatoms (Class Bacillariophyceae)Observer
laneallenDescription
All specimens from the Colorado Front Range.
Scale Bar = 10 µm.
What
Genus NeidiumObserver
laneallenDescription
NOT N. bisulcatum, except maybe the first 3. N. bisulcatum sensu strictu has a width range of 8-9 microns. The majority of the specimens here are a similar taxon that has been erroneously lumped into the species concept of N. bisulcatum by many authors over the course of the last century.
Scale bar = 10 µm.
Photos / Sounds
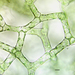
What
Water Net (Hydrodictyon reticulatum)Observer
laneallenDescription
Scale Bar = 10 µm.
What
Tardigrades (Phylum Tardigrada)Observer
laneallenDescription
Found alive and well in a culture in our incubator neglected since the onset of the pandemic. Interestingly enough, some cultures contained dead tardigrades and live rotifers. Source material was antarctic algal mats. Photo taken using darkfield.
What
Genus PsammothidiumObserver
laneallenDescription
Scale Bar = 10 µm
This is an unusually large monoraphid.
What
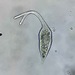
Sliminess of the Skin (Trichodina domerguei)Observer
plingfactoryDescription
found as epibionts on a copepod.
More on this here:
http://www.plingfactory.de/Science/Atlas/KennkartenProtista/01e-protista/e-Ciliata/e-source/Trichodina%20domerguei%20megamicronuleata.html
Photos / Sounds
Observer
laneallenDescription
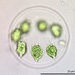
Found in an alpine rock pool. photos taken at 400x total magnification. Another type of colonial motile green algae. It appears to be morphologically distinct from the other taxa I documented here. I have a photo of the two taxa next to each other if anyone is interested.
Photos / Sounds
Observer
laneallenDescription
Found in an alpine rock pool. photos taken at 400x total magnification. This was a motile colonial organism not unlike Eudorina or Pandorina.